|
Fifth Dimension Catalog Contact Us |
||||||||

|
Impact of aging on the biology of breast cancer |
|||||||
| ||||||||
Abstract
Back to the Table of Contents
Breast cancer is a heterogeneous malignancy; its age-specific incidence profile rises exponentially until menopause and increases more slowly thereafter, reflecting the superimposition of early-onset and late-onset breast cancer rates. While early-onset breast cancers largely represent inherited or early life transforming effects on immature mammary epithelium, late-onset breast cancers likely follow extended exposures to promoting stimuli of susceptible epithelium that has failed to age normally. Among stimuli thought to promote late-onset breast tumorigenesis are the altered extracellular matrix and secreted products of senescent fibroblasts; however, the extent to which these senescent influences exist within the aging breast remains unknown. Clinical observations and biomarker studies indicate that late-onset breast cancers grow more slowly and are biologically less aggressive than early-onset breast cancers, even when controlled for hormone receptor (e.g. estrogen receptor, ER) and growth factor receptor (e.g. HER2) expression, supporting the conclusion that the biology of breast cancer is age-dependent.
Breast cancer: a heterogeneous age-associated malignancy
Back to the Table of Contents
The vast majority of human malignancies are age-associated cancers, showing incidence rates that increase exponentially with age during adulthood such that over 75% of all invasive cancers occur in susceptible populations age 55 years or older. [1] Cancer incidence in the United States (US) has been monitored since 1973 by the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI), which now collects data from 18 different SEER registries representing ~26% of the US population and reports incidence and survival rates per 100,000 adjusted to the US population's age distribution in the year 2000. While overall age-adjusted US cancer incidence rates have increased about 15% over the past three decades, breast cancer age-adjusted incidence rates have increased nearly 23% to a current level of ~130 cases per 100,000, representing ~180,000 new cases yearly. [2] Unlike the age-specific incidence profile for all cancers, that for invasive breast cancer or its precursor lesion, ductal carcinoma in situ (DCIS), shows an exponential rise until menopause (about age 50) followed by a slower rate of increase, as shown by the 1992-1997 SEER incidence curves in Fig. 1 (panel A). Consequently, about 80% of all breast cancers arise in women over age 50; and the 10-year probability of developing invasive breast cancer increases from less than 1.5% at age 40, to about 3% at age 50 and over 4% by age 70, producing a cumulative lifetime risk of 13.2% or 1 in 8. [3] When the SEER incidence data shown in panel A of Fig. 1 are broken into four clinical breast cancer subsets based on registry reported estrogen and progesterone receptor (ER, PR) status, four different age-specific breast cancer incidence curves are revealed, as shown in panel B of Fig. 1. [4] Notable are the near identical increases in age-specific incidence rates for each of the four ER/PR subsets during premenopausal years and the markedly different curve inflections near age 50; only the two ER-positive breast cancer subtypes (ER-positive/PR-positive, ER-positive/PR-negative) show ever increasing rates during postmenopausal years, while ER-negative breast cancers (ER-negative/PR-negative, ER-negative/PR-positive) show a slight decline in incidence rates after age 50. The classically recognized inflection point about menopause (Clemmesen's Hook) in the overall age-specific breast cancer incidence curve is now known to reflect the superimposition of two different rate curves, an early-onset type breast cancer with a modal age of diagnosis at ~50 years and a late-onset type breast cancer type with a modal age of diagnosis at ~70 years. Over 270,000 SEER registry breast cancer cases diagnosed across the US between 1992 and 2002, with known stage and steroid receptor status (ER, PR) and charted for seven different histopathologic invasive subtypes (ductal, tubular, lobular, medullary, inflammatory, papillary, mucinous) and three different racial origins (White, Black, Asian or Pacific Islander), were analyzed by age-density plots and a statistical mixture model to reveal that a bimodal age distribution provides a better overall fit to the incidence data than a single age density distribution model. [5] High-risk tumors (large size, positive lymph nodes, high grade, negative ER and PR) show predominantly early-onset age distributions while low-risk tumors (small size, negative lymph nodes, low grade, positive ER or PR) show predominantly late-onset age distributions at diagnosis.[5] With the exception of medullary breast cancer, which fits an early age unimodal model across all racial groups regardless of steroid receptor status, all histopathologic breast cancer subtypes demonstrate similar bimodal age density distributions within each racial group. As well, when this statistical method was applied to a separate published set of breast cancer cases molecularly subclassified by gene expression microarrays (total 122 Stanford/Norway cases) into luminal (subtypes A and B) or non-luminal (basal and HER2-positive) types, both molecular types exhibited bimodal age-at-diagnosis distributions, with the luminal cases appearing most like ER-positive late-onset breast cancers and the non-luminal cases appearing most like ER-negative early-onset breast cancers.[5]
(A)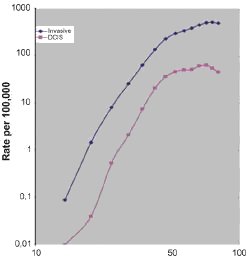
Age at DX |
(B)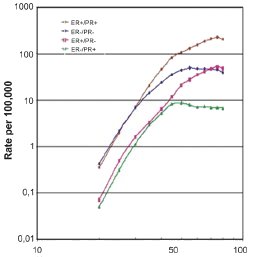
Age at DX |
Fig. 1. Age-specific incidence curves (log-log plots) for overall invasive and ductal carcinoma in situ (DCIS) newly diagnosed breast cancers (panel A), and for invasive breast cancer subsets according to ER and PR status (panel B). SEER incident rates (per 100,000, including all ethnic groups) for 5-year age groups determined from the SEER reporting interval 1992-1997. Known ER and PR status was available for over 80,000 cases across all age groups; these cases consisted of 62% ER-positive/PR-positive, 13% ER-positive/PR-negative, 4% ER-negative/PR-negative, and 21% ER-negative/PR-negative. Data and figure revised from previous publication. [4]
2. Normal mammary gland changes with aging and menopause cancer
Back to the Table of Contents
While aging is highly individualized, normal age-related changes occur in organs that are also at risk for malignant transformation. Whether these normal age-related changes represent a shifting tissue background from which malignancy must be differentiated or in some way contribute to the tumorigenic process is a fundamental question under intense investigation. Normal organ-specific aging may entail diminished tissue mass and function (e.g. liver, kidney, skeletal muscle), loss of functional reserve without substantial loss of tissue mass (e.g. cardiac muscle, lung, gastrointestinal tract, brain, marrow and immune cells, most exocrine and endocrine glands), or tissue remodeling with altered organ function (e.g. male and female reproductive glands). In non-pregnant women, ovarian size and function diminish progressively after the second decade of life, uterine size peaks by the fourth decade and then declines, and breast glandular mass is progressively lost and replaced by a combination of fatty tissue and collagenous stroma.[6,7] The molecular and cellular effects of aging on normal breast tissue are, therefore, superimposed on a continuum of developmental changes in mammary gland epithelium that normally occur between puberty and menopause, heavily influenced by menstrual history and parity. For women it is often difficult to distinguish the effects of normal aging from those of natural menopause, or the earlier incipient decline in ovarian estrogen (E) production with its resultant effects on ER expressing target organs like the breast. Expression of ER in the normal breast shows a gradual >3-fold increase beginning in the third decade and plateauing by the sixth decade of life.[4] In contrast, estrogen-inducible proteins like PR show no significant age-specific change in their average level of expression in the normal breast, although they are certainly subject to monthly changes within each menstrual cycle. Further complicating age-related influences on the normal mammary gland is the marked but variable age-related increase in breast adipose and stromal cell production of the enzyme, aromatase, encoded by the gene CYP19A1. Androstenedione and testosterone, whose serum levels in postmenopausal women are not much reduced from those in follicular phase premenopausal women, are the androgenic precursors converted by aromatase into estrone (E1) and estradiol (E2), respectively. While postmenopausal serum estrogen (E1 and E2) levels are markedly reduced relative to premenopausal serum levels, the age-related increase in mammary gland aromatase production is such that postmenopausal mammary gland estrogen levels can approach those of a premenopausal mammary gland.[8]
3. Tumorigenic predisposition within the aging mammary gland
Back to the Table of Contents
Despite longstanding awareness that breast and other cancers are primarily age-related diseases and that aging predisposes to diseases like cancer, geroscience is still in its infancy [9] and is only beginning to inform oncology about the cancer-aging relationship.[10] Consequently, emergent molecular and cellular hypotheses put forth to explain the cancer-aging relationship are of interest but remain largely untested.[11]
3.1. Timing of carcinogenic events
One obvious aspect of this relationship involves the time and number of premalignant steps required between mutagenic initiation and complete tumor promotion to generate a clinically apparent cancer. Studies of human breast cancer latency after a mutagenic dose of ionizing radiation or inheritance of a breast cancer predisposition gene (e.g. mutated BRCA1, BRCA2, TP53, ATM,or PTEN) indicate that clinical presentation generally requires decades of tumor promotion and growth. Early-onset type breast cancers showing a modal age of diagnosis at ~50 years, as determined from age-specific incidence curves, are thought to largely represent inherited or early life transforming events affecting the immature mammary epithelium.[5] In contrast, later age-onset cancers can emerge in any organ with a replicating cell subpopulation hit by an early mutagenic initiating event and then subjected to prolonged later life exposure to an exogenous or endogenous promoting agent. This later life tumor promotion can also become manifest by age-associated impairments in xenobiotic detoxification, macromolecular repair, immune surveillance or wound healing. With specific regard to breast cancer, both exogenous administration of hormones at menopause (e.g. estrogen and progesterone replacement during menopause) and specific polymorphisms in endogenous steroid hormone metabolic pathways are associated with later age predispositions to breast cancer.[12,13]
3.2. Persistence with aging of breast epithelium
susceptible to transformation
Another key aspect of the cancer-aging relationship involves the impact of development and aging on the persistence of replicating (or replication-competent) cell populations that are most susceptible to malignant transformation. For breast tumorigenesis, initiating events must occur relatively early in life since it is known that breast irradiation after age 35 fails to increase subsequent breast cancer risk, implying later-life loss of breast epithelium susceptible to full malignant transformation.[12] Epithelial cells within the human and rodent mammary gland known to be most susceptible to oncogenic transformation are the replicating and hormonally responsive subpopulations within undifferentiated terminal duct lobular units, which are normally reduced in number with increasing age and parity.[14] In a tissue reorganizing process distinct from postlactional mammary gland involution and not coincident with menopause, aging in the normal mammary gland is associated with a progressive reduction in the number and size of breast lobule acini. This loss of acinar epithelium is referred to as age-related lobular involution and is also associated with gradual replacement of the delicate intralobular stroma by a more dense collagenous breast stroma combined with variable amounts of breast fatty tissue.[7] Since the extent of age-related lobular involution is associated with a markedly reduced age-specific breast cancer risk, it has been suggested that breast cancer predisposition is closely linked to the failure of breast tissue to age and involute normally.[15] Unfortunately, the subcellular and molecular mechanisms regulating age-related mammary gland involution are still unknown; presumeably this tissue remodeling process involves some combination of programmed epithelial apoptosis and/or senescence, mechanisms for which there is a growing body of knowledge and increasing evidence of linkage to both aging and cancer. [16]
Please cite this article in press as: Benz CC, Impact of aging on the biology of breast cancer, Crit. Rev. Oncol./Hematol. (2007), doi:10.1016/j.critrevonc.2007.09.001
|
You are welcome to share this © article with friends, but do not forget to include the author name and web address. Permission needed to use articles on commercial and non commercial websites. Thank you. |